OPAL-RING
Takayuki ISHIDA Laboratory
Applications of molecular magnetic materials and rare-earth complexes
| Faculty/Department | Department of Engineering Science Graduate School of Informatics and Engineering |
|---|---|
| Members | Takayuki Ishida, Professor |
| Affiliations | Chemical Society of Japan; American Chemical Society; Division of Molecular Electronics and Bioelectronics, Japan Society of Applied Physics; Japan Society for Molecular Science; Society of Electron Spin Science and Technology; Society of Muon and Meson Science of Japan; Japan Society of Coordination Chemistry |
| Website | http://ttf.pc.uec.ac.jp/ |
As of August, 2015

- Takayuki ISHIDA
- Keyword
-
Molecular magnetic material, transition-metal complex, coordination compounds, rare-earth complex, lanthanides, magnetic properties, X-ray crystal structure analysis, genuine organic radical ferromagnet, single-molecule magnet, control of magnetic properties by molecular encapsulation, chiral magnet, optical control of magnetic properties
Summary of Research
Discovery of Approximately Half of All Known Genuine Organic Radical Ferromagnets
Honoring the founding principles of the University of Electro-Communications, our laboratory pursues studies in areas of chemistry associated with electronics. Our central research themes include the application of molecular magnetic materials and the application of rare-earth complexes.
Scientific progress has overturned one conventional tenet of organic chemistry after another. Following the elucidation of the mechanism that gives rise to the electrical conductivity of organic compounds and the discovery of organic superconducting materials, scientific interest is shifting to explore the following question: “Can organic compounds be ferromagnetic? Can they be magnets?” Since the dawn of organic chemistry, organic compounds were believed to be diamagnetic; while each electron has magnetic momentum, electrons combine into pairs, with electrons having opposite magnetic momentum forming atomic bonds and thereby canceling out the other’s magnetic moment. The molecule of an organic compound as a whole becomes diamagnetic.
This long-held belief was abandoned when a group of Japanese researchers discovered an organic material that behaved as a magnet. This discovery was followed by the discovery by a French group of another organic compound that behaved like a magnet. Our laboratory became the third group to identify an organic material that behaves in this way. Since then, our laboratory has been responsible for the discovery of half of all known genuine organic radical ferromagnets.
For all compounds, the temperature at which ferromagnetism is lost (the phase transition temperature) is low. Current efforts seek to improve this property.
At our laboratory, organic synthetic methods are mainly used to synthesize compounds, after which we measure the changes in magnetic properties when temperatures fall to 1.8 K (kelvin).
If a compound exhibits curious properties, we seek to create a single crystal of the compound, which we then seek to analyze with an X-ray crystal structure analysis system. This is followed by investigations from a theoretical perspective to elucidate the relationship between magnetic properties and the crystal structure.
Study of Transition Metal Complexes
Our laboratory also studies complexes (coordination compounds) of organic compounds and transition metal ions. Exhibiting the properties of both metals and organic compounds, complex compounds may be regarded as hybrids of organic and inorganic materials.
We recently developed a magnet exhibiting a record-breaking coercive force of 52 kOe (kilo-oersted) from a complex formed from organic radicals and cobalt (II) ions. Coercive force corresponds to the width of the magnetic hysteresis loop; the larger the value, the less likely the material will be demagnetized.
We have also synthesized a nanometer-sized tubular complex composed of radicals and copper ions. This achievement led to the discovery that we can induce ferromagnetic interactions by filling the tube with alkali halides or water. The phenomenon was especially notable for water: The tubular complex exhibited a six-fold increase in magnetization when filled with water. Simply emptying the tube reversed these effects.
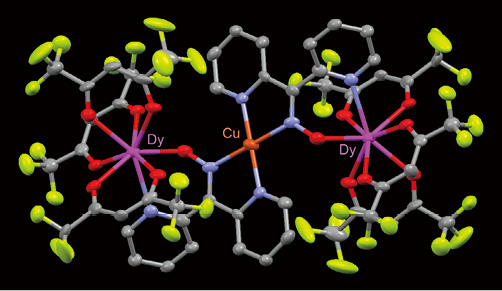
- Dy2Cu complex, a monomolecular magnet (Dy: dysprosium)
Applications of Lanthanide Complexes
Another area of interest is the application of lanthanide complexes. We have successfully synthesized a complex molecule with gigantic magnetic moment (spin quantum number: 27/2) from a complex containing both transition metal ions and lanthanide ions.
Working with a similar complex, we developed a complex that behaves as a single-molecule magnet at low temperatures. This striking magnetic material differs from conventional magnets in that a single molecule of the material exhibits magnetic hysteresis. Promising great potential for use in producing ultra-high-density information recording media, the material is expected to play a vital role in future nanotechnologies.
Since lanthanides are also important light-emitting elements, they are likely to become key materials in techniques for controlling magnetic properties with light.

- Electron spin resonance spectrometer
Advantages
Developing Magnetic Recording Media
Our laboratory is equipped with systems to manage physical properties oriented towards the development and synthesis of materials, as well as theoretical research. Joining forces with businesses that operate instruments for industrial applications (instruments for processes, vapor deposition, and silicone manufacturing), we can create effective environments for the joint development and production of magnetic recording media based on organic or organic-inorganic hybrid materials.
We offer materials based on complexes and organic radicals.
In the area of complex compounds, we offer a wide selection of single-molecule magnets with high potential for application as nanotech memory devices.
Interest in organic compounds has grown alongside their recent introduction for use as battery materials. Although radicals have long been deemed unsuitable for industrial applications due to their instability, we have proven that radicals of the appropriate molecular design can exist in a stable state, even under high temperature conditions or exposure to air.
Future Prospects
Controlling Magnetic Properties Through Molecular Encapsulation and Chiral Magnets
Our laboratory is also pioneering research on the control of magnetic properties via molecular encapsulation or chiral magnets. Using organic and molecular materials, we can develop functional composite materials with properties existing inorganic materials fail to provide. Examples include soluble, light emitting, and transparent magnets. These properties may lead to applications in the development of magneto-optical switching devices.

- X-ray crystal structure analysis system

- Superconducting quantum interference device magnetometer
