| |
Catalysis is a highly demanding technology for sustainable society and drives innovation in many other fields. Todayover 90% of all industrial chemicals are produced with the aid of catalysts. World catalyst demand is forecast to grow to$16.3 billion through 2012 and earlier global sales of catalysts is projected to be around 12 billion dollars. The catalysisof organic reactions by homogeneous and heterogeneous catalysts remains a vibrant field of scientific inquiry. Itattracts a diverse group of scientists with specialties spanning synthetic organic chemistry, inorganic chemistry, surfacescience, materials science, reaction engineering and computational modeling.
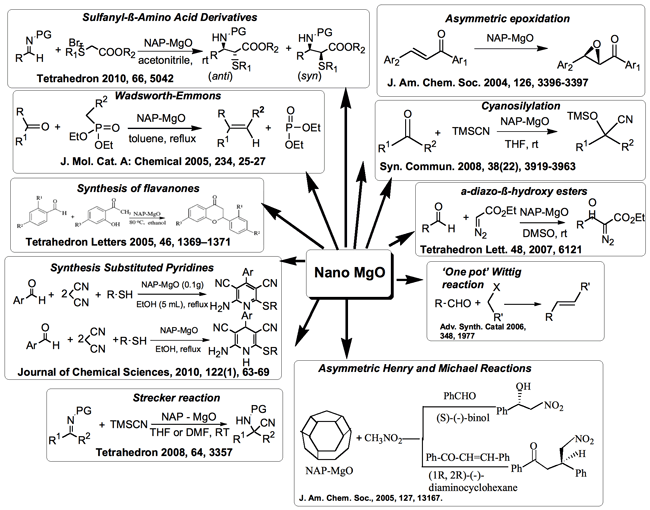
Nanomaterials are playing a significant role in diverse fields of chemistry, physics, biology and materials science.Nanostructured metal oxides are widely used in catalysis where the acidic/base properties and the catalytic activitiesare closely related to the size and morphology of the oxides. For example, heterogeneous catalysts in the form ofnanosize transition metal particles dispersed onto microporous supports have been applied to chemical conversiontechnologies for many decades. The effect of particle size has been well demonstrated in the past decade but theshape of the nanoparticles has rarely been discussed. Here we illustrate that the catalytic performance is largelydependent on the shape of the catalyst by taking nanocrystalline magnesium oxide MgO as an example.
|